How To Remove Silicon Oxide From Iron

This study investigates the removal of silica and alumina as impurities from hematite based low-grade iron ore containing 3418 mass iron 3110 mass of silica and 765 mass alumina.
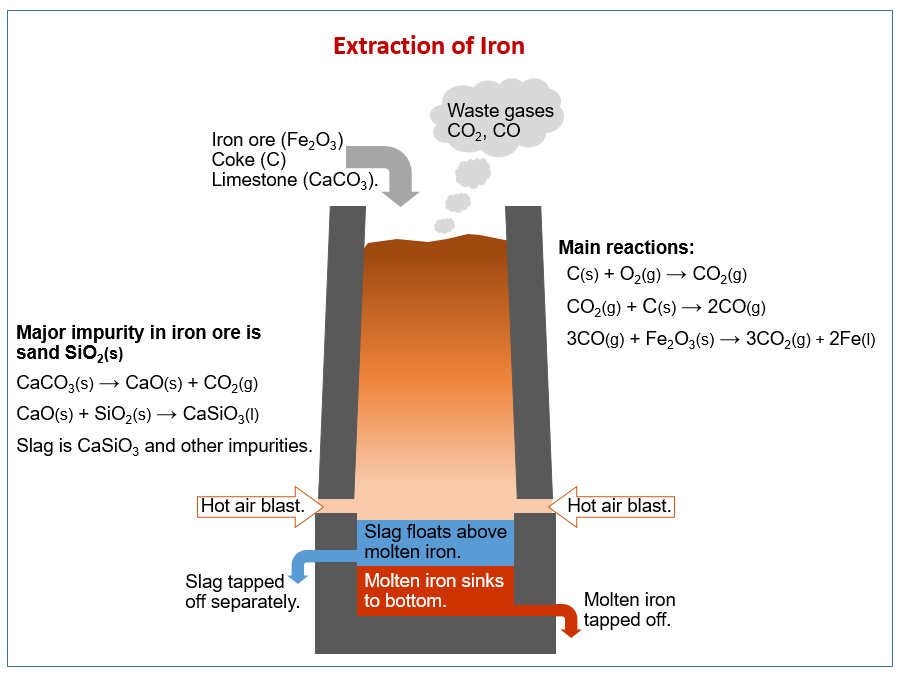
How to remove silicon oxide from iron. Calcium oxide is used to remove silicon impurity in iron extraction. Iron Ore processing for bulk commodity sales typically includes communution density and magnetic seperation to separate iron bearing minerals from. Calcium oxide also plays an important part in the Basic Oxygen Steelmaking furnaceHere it helps to remove impurities such as phosphorus and sulphur from the steel.
Silicon dioxide reacts with calcium oxide to form calcium silicate called slag which is liquid in the furnace. To iron and the carbon is oxidised. The ore is crushed into small pieces and is washed with water to remove the sand particles.
The pilot plant had continued to pro-duce rust for several weeks as long as it was run and the effluent was highly colored with finely divided hydrous. Factory price biogas h2s scrubber Iron Oxide desulfurizer to remove H2S for Biogas Purification Biogas scrubberMore information please contact with us Mob. The removal of alumina and silica from iron rejects slime Jan 01 2011 A hydrometallurgical method of alumina and silica gangue removal from rejects slime of iron ore by alkali and acid leaching is proposed Up to about 80 gangue is removed by chemical leaching with sodium hydroxide sulphuric hydrochloric and nitric acids.
In WHIMS process 9308 of iron was recovered with a grade of 5322 mass at an optimum. Tartaric acid citric acid and acetic acid. Thus for example Knowles et al in US.
This CaO later combines with silica and forms slag. Be careful not to get a thermal shock while doing this. Using magnesium compounds during the hot lime-soda process of softening and recirculating the sludge.
Extraction of Iron is done in many steps like oxidation combustion fusion reduction etc. After magnetic separation to remove the remainder of the liberated particles it was found that the resulting concentrate had a silica content of 4. 2Fe 2 O 3 s 3Cs 4Fel 3CO 2 g In this reaction the ironIII oxide is reduced.